Dynamic light scattering
Dynamic light scattering is the measurement of fluctuations in scattered light intensity with time. These fluctuations in intensity arise due to the random Brownian motion of the nanoparticles. Therefore, the statistical behavior of these fluctuations in scattered intensity can be related to the diffusion of the particles. Since larger particles diffuse more slowly than small particles one can readily relate particle size to the measured fluctuations in light scattering intensity. With modern instruments such as the SZ-100 the technique is rapid and reliable.
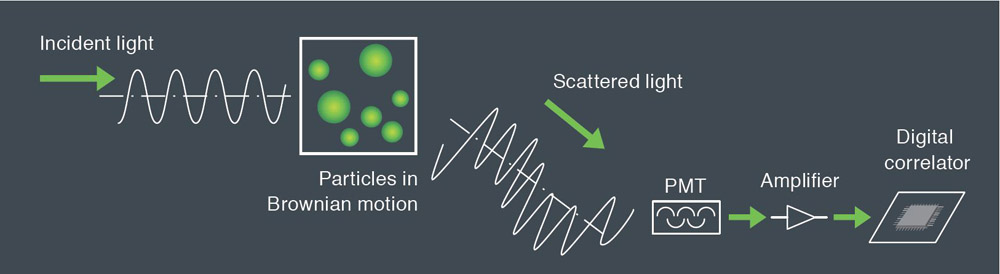
Larger particles in Brownian motion have smaller rate than smaller. The scattered light from the particles is detected as a signal to the fluctuations corresponding to speeds of Brownian motion of particles. The resulting signal is analyzed using correlation spectroscopy, the autocorrelation function is calculated and based on this construction the particle size distribution. The relationship between the autocorrelation function and the diameter of the nanoparticles:
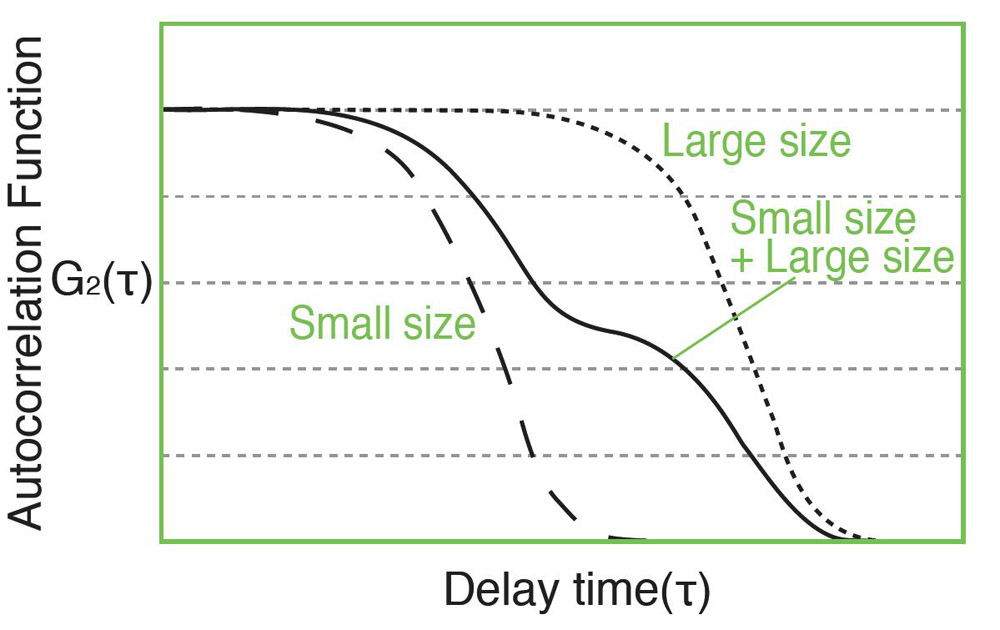
Measurement of the autocorrelation function is done by comparing the scattered light intensity at some reference time t and after some delay time t. For a very short delay time. The particles have not had a chance to move and therefore the scattered light intensity is unlikely to change much. So, the autocorrelation function has a high value. For a very delay time, the particles have had a chance to move significantly, and the autocorrelation function has a low value. This low value is related to the time average scattered intensity. The rapidity of this decay from high values to low values corresponds to the speed of particle motion and therefore to the particle size. The measured autocorrelation function has an exponential decay.
Overviews and methods
Under construction
Equipment
Laser particle analyzer SZ100
Contacts
The Leading Researcher in optical systems Alexandr Shimko
Specialist in spectroscopy and granulometry Anastasia Povolotckaia
Specialist in optical systems Alexandra Mikhaylova

 English (UK)
English (UK)  Русский (РФ)
Русский (РФ)